Our Technologies
Catalytic and Redox Solutions has obtained licenses for technologies from NC State University that focus upon the conversion of natural/shale gas and other hydrocarbons to value added products using chemical looping and catalytic technologies. By using highly tailored redox catalyst for chemical looping we can drive low temperature selective oxidation of low value hydrocarbons into more valuable produces, such as syngas and olefins. Chemical looping allows us to perform these reactions at low temperatures while avoiding the use of oxygen co-feeds. This makes these technologies ideal for distributed scale implementation. Technologies license by us include energy efficient chemical looping materials and processes
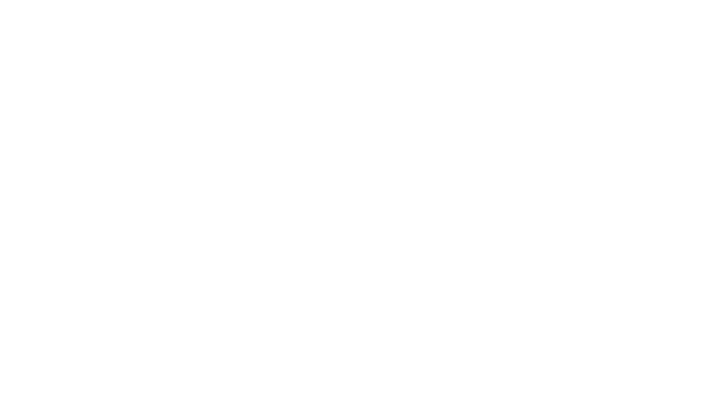
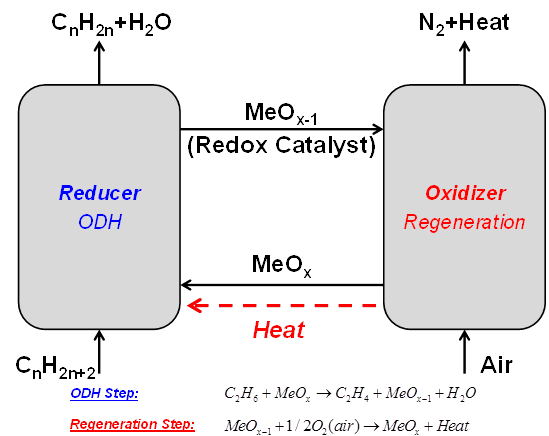
Oxidative dehydrogenation light alkanes to alkenes e.g. ethane to ethylene
The partial oxidation of hydrocarbons to yield alkenes and oxygenates, which are raw materials for a large diversity of processes, is of a high economic importance worldwide. In the chemical industry, oxidation reactions of hydrocarbons are associated with essential processes thus playing a key role in a large diversity of industrial, environmental and energy applications. Among the partial oxidation reactions investigated to date, the catalytic oxidative dehydrogenation (ODH) of light alkanes is, undoubtedly, one of the most promising technologies for the production of alkene species.
Shale gas liquefaction at distributed scales
Through the small-scale Distributed LNG Production, we reduce the size of the gas volume by 600 times, facilitating its transportation through the Virtual Pipeline. Hence, a direct connection between gas sources and the people who benefit the most from a clean and cheap fuel – LNG, is established. This is how we offer the possibility to monetize resources which previously lacked value, or to expand the reach of infrastructure reducing CAPEX.

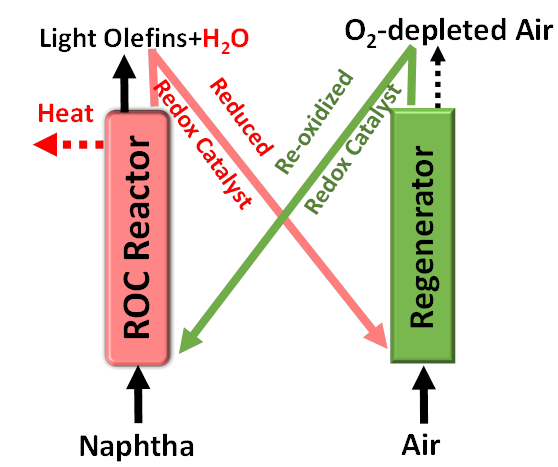
Oxidative cracking of light naphtha and natural gas condensates
As propylene market is expanding, new production paths have to be found. The cracking of light olefins contained in several naphthas seems to be a good alternative for responding to this demand. Results of light FCC naphtha cracking have shown that selectivity towards propylene is governed by hydrogen-transfer reactions. Thus, the selectivity to propylene may be increased by minimizing these reactions. This can be achieved by cracking the naphtha at high temperature, by using shape-selective catalysts or by working with coked catalysts. Recycling light naphtha in the FCC process is an interesting alternative, which may increase the yield of propylene by 50% if proper processing is carried out. Furthermore, olefins content of FCC gasoline may be significantly reduced.
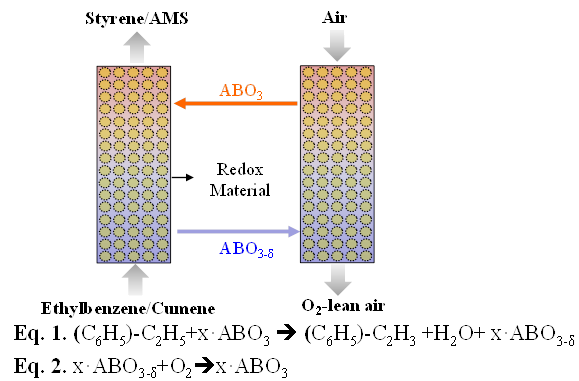
Dehydrogenation of alkyl benzenes such as ethyl benzene and cueme
The direct alkylation can not be performed because there is polysubstituion product is formed. Due to disadvantage of polysubstituion that Friedel-Craft’s alkylation reaction is not used for preparation of alkylbenzenes. Instead of Friedel-Craft’s acylation is used. The problem with more complicated alkenes like propene is that you have to be careful about the structure of the product. In each case, you can only really be sure of that structure if you work through the mechanism first.
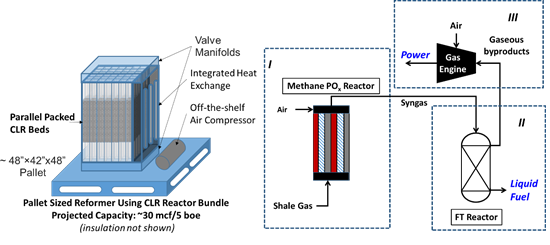
Low-temperature production of Fischer-Tropsch or methanol ready syngas from methane
Synthesis gas is a very important chemical intermediate for many relevant processes including the production of methanol and Fischer-Tropsch synthesis of synthetic fuels. Syngas is currently produced by the endothermic steam reforming of methane or by homogeneous oxidation in autothermal reforming.
